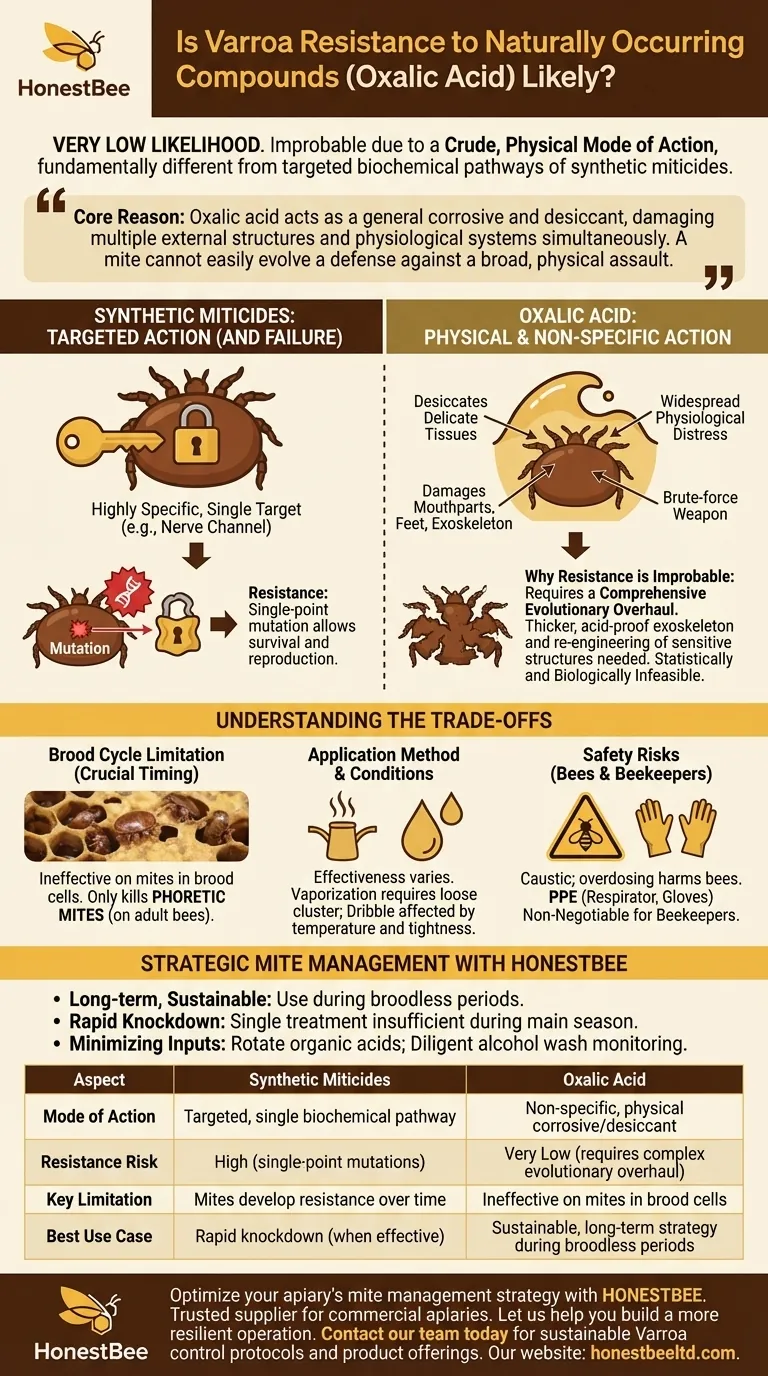
In short, the likelihood is very low. It is widely considered improbable that Varroa mites will develop a meaningful resistance to organic acids like oxalic acid. This is because these compounds attack the mites through a crude, physical mode of action, which is fundamentally different from the targeted biochemical pathways attacked by synthetic miticides.
The core reason resistance is unlikely is that oxalic acid acts as a general corrosive and desiccant. It damages multiple external structures and physiological systems simultaneously. A mite cannot easily evolve a defense against such a broad, physical assault, unlike the single-point mutations that can overcome a targeted synthetic chemical.

The Core Difference: Physical vs. Targeted Action
To understand why resistance is improbable, we must first distinguish between the two major ways miticides work. The entire story of resistance lies in this distinction.
How Synthetic Miticides Work (and Fail)
Most synthetic miticides (like pyrethroids or organophosphates) are highly specific. They are designed to attack a single, precise target, such as a particular nerve channel or a critical enzyme in the mite's body.
This specificity is also their weakness. A small, random genetic mutation in a few mites can alter the shape of that one target. Those mites survive the treatment, reproduce, and pass on their genetic advantage, leading to a resistant population.
How Oxalic Acid Works
Oxalic acid has a non-specific, physical mode of action. As a strong acid, it attacks the mite on multiple fronts upon contact.
It primarily functions by desiccating (drying out) the mite's delicate tissues. It is also believed to damage the mite's mouthparts, feet (empodia), and exoskeleton, causing widespread physiological distress. It is a brute-force weapon, not a surgical one.
Why This Prevents Resistance
For a mite to "resist" oxalic acid, it wouldn't need a simple mutation. It would need a comprehensive evolutionary overhaul.
The mite would have to develop a fundamentally thicker, acid-proof exoskeleton and completely re-engineer the sensitive structures it uses to walk and feed. This would require numerous, complex genetic changes to occur simultaneously, which is statistically and biologically infeasible.
Understanding the Trade-offs
While resistance is not a concern, oxalic acid is not a perfect solution. Its effectiveness is highly dependent on how and when it is used.
The Brood Cycle Limitation
This is the most significant limitation of oxalic acid. It does not kill mites reproducing under the wax cappings of brood cells.
It is only effective against phoretic mites—those physically present on the bodies of adult bees. This makes timing a critical factor for success.
Application Method and Conditions
The effectiveness of oxalic acid is tied to the application method (vaporization, dribble) and ambient conditions.
Vaporization, for example, requires the bee cluster to be loose enough for the vapor to circulate. The dribble method can be less effective or more harmful to bees if applied incorrectly or in cold temperatures when the cluster is tight.
Safety Risks for Bees and Beekeepers
Though considered "organic," oxalic acid is a caustic substance. Overdosing or too-frequent applications can harm the bees' cuticles and digestive systems.
For the beekeeper, it is a hazardous material. Proper personal protective equipment (PPE), including an acid-rated respirator, gloves, and eye protection, is non-negotiable to prevent serious injury to the lungs and skin.
Making the Right Choice for Your Mite Strategy
Your treatment decisions should be based on a clear understanding of your goals and the limitations of each tool in your arsenal.
- If your primary focus is a long-term, sustainable plan: Use oxalic acid as a cornerstone treatment during broodless periods, such as in late fall or by creating an artificial brood break.
- If your primary focus is a rapid knockdown of high mite loads: Recognize that a single oxalic acid treatment will be insufficient during the main season, as it will not affect the majority of mites reproducing in the brood.
- If your primary focus is minimizing chemical inputs: Rely on a rotation of organic acids like oxalic and formic, but be absolutely diligent about monitoring mite levels with alcohol washes to confirm your treatments are working.
By understanding why oxalic acid is a reliable long-term tool, you can make strategic decisions for the sustained health of your colonies.
Summary Table:
| Aspect | Synthetic Miticides | Oxalic Acid |
|---|---|---|
| Mode of Action | Targeted, single biochemical pathway | Non-specific, physical corrosive/desiccant |
| Resistance Risk | High (single-point mutations) | Very Low (requires complex evolutionary overhaul) |
| Key Limitation | Mites develop resistance over time | Ineffective on mites protected in brood cells |
| Best Use Case | Rapid knockdown (when effective) | Sustainable, long-term strategy during broodless periods |
Optimize your apiary's mite management strategy with HONESTBEE.
As a trusted supplier for commercial apiaries and beekeeping equipment distributors, we understand the critical need for effective, long-term solutions. Our wholesale-focused operations provide the reliable supplies and equipment you need to implement sustainable Varroa control protocols.
Let us help you build a more resilient operation. Contact our team today to discuss your needs and explore our product offerings.
Visual Guide
Related Products
- Adjustable Formic and Acetic Acid Dispenser for Bee Mite Treatment
- Varroa Easy Check Mite Tester Kit Counter Alcohol Wash Jar
- Economy Galvanized Beekeeping Honey Bee Smoker for Wholesale
- Professional Bee Smoker with Elongated Spout and Durable Bellows for Beekeeping
- 8-Frame Electric Self-Reversing Honey Extractor Spinner for Commercial Honey Extraction Equipment
People Also Ask
- Why is a high-precision larva and pupa extraction process required when analyzing Varroa mite reproductive success?
- How does a precision evaporative formic acid dispenser treat Varroa mites? Master Controlled Pest Management
- What is the application method for cardboard-based Varroa mite treatments? Maximize Hive Health with Correct Placement
- What are the technical requirements for Varroa mite treatments? Essential Strategies for Colony Health
- What are the common technical treatments used for Varroa mite control in the spring? Optimize Colony Health Today