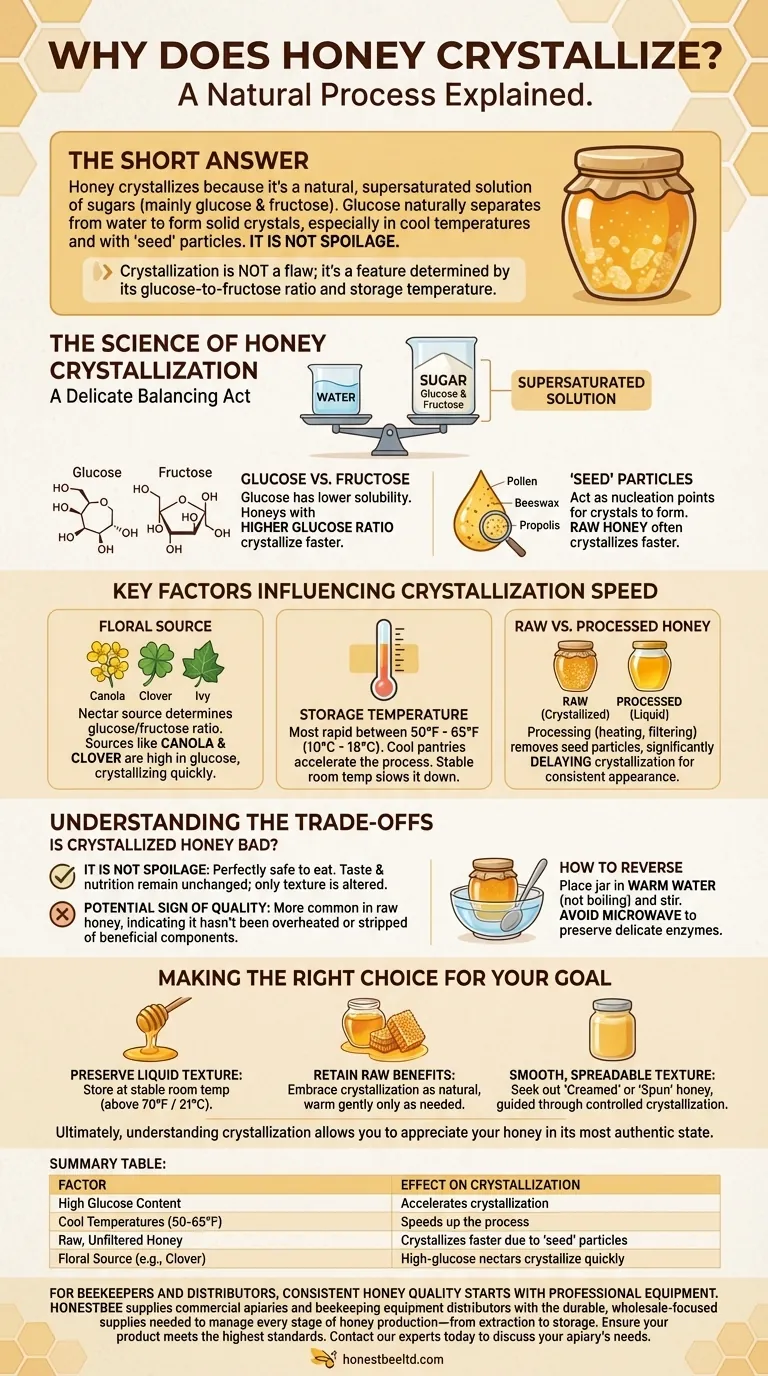
The short answer is this: Honey crystallizes because it's a natural, supersaturated solution of sugars, primarily glucose and fructose. The glucose is prone to separating from the water and forming solid crystals, a process accelerated by cooler temperatures and the presence of tiny particles like pollen. This is a natural transformation, not a sign of spoilage.
The core takeaway is that crystallization is not a flaw in your honey; it's a feature. This natural process is driven by the honey's specific glucose-to-fructose ratio, which is determined by the flowers the bees visited, and by its storage temperature.

The Science of Honey Crystallization
To understand why honey crystallizes, it helps to think of it as a delicate balancing act between sugar and water.
A Supersaturated Sugar Solution
Honey is fundamentally a solution containing more sugar than can normally be dissolved in its small amount of water. This supersaturated state is inherently unstable, making it prone to change over time. The excess sugar, specifically glucose, will eventually solidify.
The Role of Glucose vs. Fructose
Honey is composed of two main sugars: glucose and fructose. Glucose has lower solubility in water compared to fructose. Therefore, honeys with a higher ratio of glucose to fructose will crystallize much more quickly and solidly.
"Seed" Particles as a Starting Point
In raw, unfiltered honey, small particles like specks of pollen, beeswax, and propolis are present. These particles act as "seeds," or nucleation points, giving the glucose crystals a surface to form on. This is why raw honey often crystallizes faster than highly processed commercial honey.
Key Factors That Influence Crystallization Speed
Not all honey crystallizes at the same rate. Three primary factors control how quickly this natural process occurs.
The Floral Source
The nectar source is the single most important factor. Nectar from different flowers has a unique glucose-to-fructose ratio. Honeys from sources like canola, clover, ivy, and goldenrod are naturally high in glucose and can begin to crystallize within weeks.
Storage Temperature
Crystallization happens most rapidly at temperatures between 50°F and 65°F (10°C and 18°C). Storing honey in a cool pantry can actually accelerate the process. Storing it at a stable room temperature or in a warm place will keep it liquid longer.
Raw vs. Processed Honey
Commercially processed honey is often heated and ultra-filtered. This process dissolves any existing sugar crystals and removes the "seed" particles like pollen, which significantly delays the onset of crystallization for a more consistent shelf appearance.
Understanding the Trade-offs: Is Crystallized Honey Bad?
It's crucial to distinguish between a natural physical change and spoilage. Crystallization is a physical change, not a chemical one.
It Is Not Spoilage
Crystallized honey is perfectly safe to eat. The taste and nutritional properties remain unchanged. The only thing that has been altered is the texture.
A Potential Sign of Quality
Because the process is more common in raw, unfiltered honey, many enthusiasts view crystallization as a sign of high-quality, natural honey. It indicates that the honey hasn't been overheated or stripped of its natural components, including beneficial pollen.
How to Reverse Crystallization
If you prefer your honey in a liquid state, the process is easily reversible. Simply place the honey jar in a bowl of warm (not boiling) water and stir it occasionally until the crystals dissolve. Avoid using a microwave, as it can overheat the honey and degrade its delicate flavors and beneficial enzymes.
Making the Right Choice for Your Goal
Your approach to crystallization should depend on what you value most in your honey.
- If your primary focus is preserving a liquid texture: Store your honey at a consistent room temperature (above 70°F / 21°C) and be aware that honeys from high-glucose sources like clover will solidify regardless.
- If your primary focus is retaining the benefits of raw honey: Embrace crystallization as a natural sign of an unprocessed product and gently warm only the amount you need at a given time.
- If your primary focus is a smooth, spreadable texture: Seek out "creamed" or "spun" honey, which is honey that has been guided through a controlled crystallization process to create very fine, smooth crystals.
Ultimately, understanding crystallization allows you to appreciate your honey in its most authentic and natural state.
Summary Table:
| Factor | Effect on Crystallization |
|---|---|
| High Glucose Content | Accelerates crystallization |
| Cool Temperatures (50-65°F) | Speeds up the process |
| Raw, Unfiltered Honey | Crystallizes faster due to 'seed' particles |
| Floral Source (e.g., Clover) | High-glucose nectars crystallize quickly |
For beekeepers and distributors, consistent honey quality starts with professional equipment. HONESTBEE supplies commercial apiaries and beekeeping equipment distributors with the durable, wholesale-focused supplies needed to manage every stage of honey production—from extraction to storage. Ensure your product meets the highest standards. Contact our experts today to discuss your apiary's needs.
Visual Guide
Related Products
- Hexagonal Glass Honey Jars with Metal Lug Caps Elegant Versatile Packaging
- Squeezable No-Drip Beehive-Shaped Honey Jars with Flip-Top Cap
- Inverted Squeezable Honey Jar with No Drip Flip Top Cap for Easy Pouring
- Classic Drum Shaped Glass Honey Jar with Airtight Lid
- Classic Honey Bear Jars with Flip Top Dispensing Cap for Liquid Sweeteners
People Also Ask
- What is the best way to jar honey? Preserve Quality with the Right Container
- What is done with the honey after extraction and filtering? From Purification to Perfect Packaging
- Why are Glass Jars preferred as storage equipment for honey? The Ultimate Choice for Purity and Long-Term Quality
- What key functions do high-quality packaging materials serve? Unlock the Commercial Potential of Your Honey
- What are the primary functional benefits of high-standard glass packaging for honey? Protect Purity and Brand Value



















